Vitamin C: Prevention of Chronic Diseases and Optimal Doses
Abstract
The role of vitamin C in prevention and treatment of scurvy is well accepted. In spite of having long history as the candidate of alternative therapy for the prevention and treatment of cancer, still there is no common conclusion on the topic. However, its biochemical reaction as an antioxidant and its immunostimulating effects drew further attention towards its health beneficial effects. Current official recommended dietary allowance in most of the countries is higher than what is needed for the prevention of scurvy, but research result suggested that it is far less to obtain optimum health. Therefore, especially for the prevention of chronic illness such as cancer, hypertension, Alzheimer’s diseases etc., it is recommended to consume more fruits and vegetables together with the vitamin C supplements. Evaluating the vitamin C efficiency and safety with current literature, up to 1 g/day supplement of vitamin C has been suggested to be needed for optimal health. In addition, male population compared to female population, older population compared to younger population, smoking population compared to non-smoking population and stressed population compared to non-stressed population need larger amounts of vitamin C to obtain health beneficial effects.
Main message
Vitamin C (ascorbic acid) is one of the essential components in human physiology working as a water-soluble antioxidant and enzyme cofactor. Humans do not synthesize ascorbate, therefore we need to administer it through diet or supplements. We must assure a sufficient intake of vitamin C in order to define its role for the prevention or treatment of various diseases.
Download article in pdf-format
INTRODUCTION
It has been known for a long time that absence of fresh fruit and vegetables in the human diet leads to scurvy, a fatal disease widely described throughout written history (Lind, 1753). Later, it was discovered that ascorbic acid in fresh fruits and vegetables prevents scurvy and that is why it is a vitamin for humans and must be a part of human diet (Mandl et al., 2009).
Among the long list of people who contributed to the knowledge regarding vitamin C, three Nobel prize-winners are in the fore front. Albert Szent-Györgyi discovered vitamin C as an anti-scorbutic factor (Svirbely and Szent-Györgyi, 1932). Walter Norman Haworth used the material from Szent-Györgyi and elucidated the structure and synthesized vitamin C (Haworth and Hirst, 1933). For their contributions, Szent-Györgyi and Haworth were rewarded with Nobel prizes in medicine and chemistry in 1937, respectively. The third person, Linus Pauling is perhaps the most renowned advocate of vitamin C. Pauling believed that high doses of vitamin C can cure or prevent several mental illnesses, chronic diseases such as heart diseases and cancer, including common cold (Pauling, 1970; Cameron and Pauling, 1973; Cameron and Campbell, 1974; Pauling, 1974; Cameron and Pauling, 1979; Cameron and Pauling, 1993). The knowledge contributed by these three great scientists and many others led to the foundation for research on vitamin C.
Although the role of vitamin C in the prevention and treatment of scurvy is well accepted, immunomodulatory effect of vitamin C, its role as an antioxidant to prevent or treat chronic illness and official recommended dietary allowance (RDA) are under critical discussion (Deruelle and Baron, 2008; Mandl, 2009). In this review, the effect of vitamin C on cancer and other diseases and optimal doses are discussed.
Unique Chemical Feature and Biological Function of Vitamin C
Vitamin C (C6H8O6) is named as ascorbic acid because of its anti-scorbutic property (Latin word scorbutus = scurvy). In solution, it releases a proton to give an anion called ascorbate. Ascorbate easily transfers one electron and one additional proton and can remains in the stable radical form as semidehydroascorbate (SDA), a state in between dehydroascorbic acid (DHA) and ascorbate in the physiological condition (figure 1).
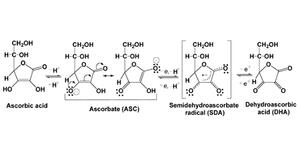
Figure 1. Structure of ascorbic acid and its fates during physiological reactions.
Because of the unique electron transferring capability, ascorbate plays a vital role in living cells. Our body synthesizes collagen to strengthen connective tissue. For this, prolyl residue must be hydroxylated and the reaction is carried out by the enzyme prolyl hydroxylase with the support of vitamin C (figure 2). The hindrance of this biochemical reaction is the cause of scurvy. In spite of having its vital role in physiology, human is unable to synthesize ascorbate due to the absence of the enzyme, gulonolactone oxidase (GLO) as found in most animals and those animals including human unable to produce GLO, need external supplement of ascorbate as vitamin to carry out normal physiological functions (Linster and Van Schaftingen, 2007).
Besides acting as a cofactor in several metabolic reactions, it serves primarily as a biologic antioxidant and free radical scavenger in aqueous environment. Free radicals produced by enzymatic and nonenzymatic reactions inside and outside cells have been suggested as a major cause of aging process (Harman, 1956; Harman, 1994). This theory was further supported by the discovery of superoxide dismutase (SOD) (McCord and Fridovich, 1969). Since then, a great deal of evidences has been accumulated implicating free radical reactive oxygen and nitrogen species in the pathology of a number of chronic diseases and age associated functional decline (Knight, 1998, Ratten 2006; Viña et al., 2007; Gruber et al., 2008; Ljubunic and Reznick 2009). Ascorbate readily scavenges many physiologicallyrelevant reactive oxygen and nitrogen species and is the most effective endogenous aqueous phase antioxidant in human plasma under many different oxidizing conditions (Frei et al., 1989). Although other endogenous antioxidants are able to decrease the rate of lipid and protein oxidation in plasma, only ascorbate is reactive enough to intercept oxidants before they can cause detectable oxidative damage. Due to its role as an antioxidant and by having immunomodulatory functions, vitamin C is one of the most promising molecules of health beneficial effects. For this purpose, more than 86,000 metric tons of vitamin C was consumed only in 2003 which is more than 50 % of all the other vitamins. In addition, consumption rate is expected to increase by more than 2000 metric tons per year (Asard et al., 2004).
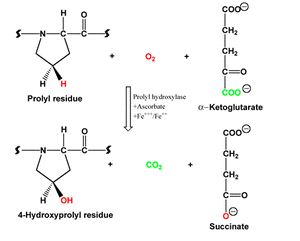
Figure 2. Hydroxylation of a prolyl residue with the enzyme prolyl hydroxylase. In this reaction, vitamin C acts as an antioxidant by reducing ferric ion which activates prolyl hydroxylase. Hydroxyprolyl residue stabilizes the collagen triple helix by forming interstrand hydrogen bonds.
Recommended Dietary Allowance for Vitamin C
The US Food and Nutrition Board has prepared RDAs for vitamin C since 1941. Initially, the RDA was based on the amount needed to prevent people from getting scurvy with a safety margin but numerous research results suggested that this may not be sufficient for optimal health (Pauling 1974; Levine et al., 1996; Food and Nutrition Board, 2000). Moreover, the RDA for vitamin C is based on estimates of rates of absorption, depletion, turnover and catabolism (Levine et al., 1999; Food and Nutrition Board, 2000). For vitamin C, however, the information is unavailable, incomplete, or flawed. Currently, RDA requirements for vitamin C differ among countries, with the highest value being 110 mg/day (Food and Nutrition Board 2000; Levine et al., 2001). According to Norwegian Social and Health Affair, the RDA for vitamin C are 75 mg per day for the adult and 100 mg per day for the women during lactating period. Pauling suggested the daily intake of vitamin C of 250–4000 mg (Pauling, 1970; Pauling 1974). Challem concluded that the RDAs might be seriously inadequate guidelines for health (Challem, 1999). However, high doses of vitamin C, as proposed by previous authors, are not supported by all literature. A meta-analysis on a potential effect of vitamin C on the common cold showed that there seems no justification for routine mega dose vitamin C supplementation, 1–3 g/day, in the normal population (Hemilä et al., 2007a). Moreover, without reaching the mega doses of vitamin C consumption, numerous reviews suggest that intakes of vitamin C much higher than the RDA may reduce the risk or risk factors for chronic diseases such as heart disease and certain types of cancer (Hathcock, 1997; Ames 2004; Ames 2005; Ames 2006). In this connection, two studies demonstrated that current RDA for vitamin C should be re-evaluated and increased to 200 mg daily (Levine, 1996; Graumlich et al., 1997).
Role of Vitamin C in Cancer Therapy
For the prevention of cancer, the US department of Agriculture and the National Cancer Institute has recommended five servings of fruits and vegetables daily. Further analyses have suggested that this consumption should be even higher (Lachance and Langseth, 1994; Guenther et al., 2006). If these recommendations are based on vitamin C and followed, daily intake will be 210 to 280 mg, depending on food type (Levine, 1999). Reports suggest that fresh produce or juice may loose 50–100 % of its vitamin C content due to handling and processing (Severi et al., 1998; Gil et al., 1999; Johnston and Bowling, 2002). Furthermore, more than 500 mg/day ofvitamin C would be difficult to obtain from dietary sources alone and therefore would require supplements especially for the prevention of cancer (Levine et al., 1995). In spite of wide use of vitamin C as an alternative therapy for the prevention and treatment of cancer, the potential cancer-therapeutic activity of vitamin C has a long and controversial history (Verrax and Calderon, 2008).
In 1973, Pauling and Cameron postulated that vitamin C inhibits tumor growth with the treatment of high doses (Cameron and Pauling, 1973). Cameron and Campbell reported beneficial effect of vitamin C based on the response of 50 consecutive patients with advanced cancer to continuous i.v. infusions (5–45 g/day) and/or oral doses (5–20 g/day) (Cameron and Campbell, 1974). Cameron and Pauling compared survival time between 100 patients with terminal cancer treated with i.v. and oral vitamin C, usually 10 g/day and 1,000 comparable patients not given vitamin C (Cameron and Pauling, 1976). Patients treated with vitamin C survived approximately four times longer than controls. A follow-up study reported that patients given vitamin C had a mean survival time almost 1 year longer than matched controls (Cameron and Pauling, 1978).
The National Cancer Institute sponsored two randomized, placebo-controlled, double-blind trials with vitamin C and advanced cancer at the Mayo Clinic (Creagan et al., 1979; Moertel et al., 1985). In both trials, patients were given 10 g/day vitamin C or placebo. Survival rates were essentially the same for all groups. Plasma concentrations of vitamin C were not measured in these studies and vitamin C was given only orally. In retrospect, the Mayo Clinic trials may have failed to properly evaluate the clinical efficacy of vitamin C in cancer because of insufficient plasma concentrations of vitamin C attained with oral supplementation (Padayatty et al., 2004).
In spite of several controversies, two phase I clinical trials with vitamin C have recently been published that demonstrated remarkable tolerance and safety for high i.v. doses up to 1.5 g/kg in patients (Riordan et al., 2005; Hoffer et al., 2008). Additionally, a series of case reports indicated that high-dose i.v. vitamin C was associated with long-term tumor regression in three patients with advanced renal cell carcinoma, bladder carcinoma, or B-cell lymphoma (Hoffer et al., 2008).
Vitamin C can be taken i.v. or orally. Oral absorption of vitamin C can not achieve plasma concentrations comparable to those obtained by i.v. administration (Padayatty et al., 2004). Intravenous doses were used as an alternative therapy to treat patients with advanced cancer (Cameron and Pauling, 1993; Padayatty et al., 2004; Riordan et al., 2005; Hoffer et al., 2008). This can be explained by the fact that i.v. doses raise plasma concentrations as high as 14,000 µmol/L, with doses of 50–100 g/day and concentrations of 1000–5000 µmol/L were found selectively cytotoxic to tumor cells but not to normal cells in vitro (Benade et al., 1969; Bram et al., 1980; Leung et al., 1993; Riordan et al., 1995; Casciari et al., 2001; Padayatty et al., 2006).
A rosy picture came forward with the recent series works of Chen et al. which showed that high doses (pharmacologic doses) of vitamin C decreased the growth and weight of human, rat, and murine tumor xenografts in athymic nude mice (Chen et al., 2005; Chen et al., 2007; Chen et al., 2008). The results suggested that millimolar concentrations of extracellular vitamin C selectively kill cancer cells but not normal cells in a hydrogen peroxide (H2O2)-dependent manner. Such millimolar concentrations of vitamin C can be achieved in humans by i.v. infusion but not by diet or supplements (Padayatty et al., 2004). Hence, vitamin C is postulated to exert local pro-oxidant effects in the interstitial fluidsurrounding tumor cells, killing them or inhibiting their growth, while leaving normal cells intact.
According to Frei and Lawson, ascorbate (ASC–), which is regarded as anti-oxidant in general, can act as a pro-oxidant by donating an electron to redox-active transition metal ions, such as ferric (Fe3+) or cupric (Cu2+) ions, reducing them to ferrous (Fe2+) or cuprous (Cu+) ions, respectively (Eq. 1) (Frei and Lawson, 2008). In fact, reduction of iron or copper in the catalytic site of certain enzymes underlies ascorbate’s well known biological function as a co-substrate in procollagen, carnitine, and catecholamine biosynthesis (Englard and Seifter, 1986). Reduced transition metal ions, in contrast to ascorbate, readily react with O2, reducing it to superoxide radicals (Eq. 2), which in turn dismutate to form H2O2 and O2 (Eq. 3) (figure 3).
In addition, the reaction of ascorbate with iron and H2O2 produces the extremely reactive pro-oxidant hydroxyl radical (Halliwell 1987). Although scientific rationale for the production of H2O2 and radicals in initiation with ascorbate as shown in figure 3 might be a matter of discussion, it is evident that ascorbate causes cancer cells to undergo apoptosis, pyknosis, and necrosis by H2O2-dependent pathways (Chen et al., 2005). In contrast, normal cells are considerably less vulnerable to H2O2. The reason for the increased sensitivity of tumor cells to H2O2 is not clear but may be due to lower antioxidant defences (Oberley and Oberley, 1997). Whatever may be the exact mechanism, the increased sensitivity of tumor cells to H2O2may provide the specificity and «therapeutic window» for the antitumor effect of extracellular millimolar concentration (higher doses) of vitamin C.
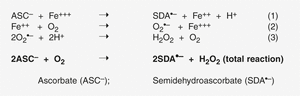
Figure 3. The possible pathways of vitamin C to generate H2O2 by the reduction of Fe+++ (Frei and Lawson).
Immune Response and other Biological Functions of Vitamin C
White blood cells store higher quantities of vitamin C even when levels in the surrounding plasma are low (Hornig, 1975; Omaye et al., 1986; Moser, 1987; Jacob, 1990). In fact, vitamin C has been defined as a stimulant of leukocyte function, especially of neutrophil and monocyte movement (Maggini et al., 2007). Vitamin C supplementation was shown to improve many indices of human immune responses, such as antimicrobial and natural killer cell activities, lymphocyte proliferation, chemotaxis, and delayed-type hypersensitivity response, and the concentration of vitamin C is found to be higher in activated neutrophils and macrophages (Wolf, 1993; Jacob et al., 1991; Washko et al., 1991; May et al., 2005). Moreover, in older people (over 70 years), known to have reduced vitamin C plasma and leukocyte concentrations, vitamin C supplementation (500 mg/day for 1 month) enhanced the proliferative response of T lymphocytes (Kennes et al., 1983). Furthermore, in healthy people and neutrophil motility defective patients, with higher concentrations of ascorbate (supplementation of 1 g/day), white blood cells became more active and could move toward infection or inflammation more quickly (Anderson, 1981; Anderson, 1981a; Anderson, 1982).
Lenton et al. demonstrated that 13 weeks with vitamin C supplementation (500 or 1000 mg/day) promoted an increase in lymphocyte glutathione level, suggesting that vitamin C may also be of value in the treatment of glutathione deficiency (Lenton et al., 2003). For example, during aging, ascorbate andglutathione in lymphocytes were found to be decreased, and low concentrations caused a higher risk of cancer, particularly lung cancer, and chronic illnesses such as ischemic heart disease, diabetes, cataract, chronic renal failure, and leukaemia (Kharb et al., 2000; Loria et al., 2000).
Men with low serum ascorbate concentrations may have an increased risk of mortality (Loria et al., 2000). Fletcher et al. have measured that men (mean age 80 years) with 100 mg/day vitamin C intake presented a mortality risk nearly half that compared to men with a consumption of 50 mg/day (Fletcher et al., 2003). A large body of evidence demonstrates that increased dietary vitamin C intake can enhance resistance to and improve recovery from infectious, degenerative diseases, and certain types of cancer (Hemilä, 1992; Hoffer and Pauling, 1993; Carr and Frei, 1999). Many biological, clinical, and epidemiological studies have indicated that higher intakes of vitamin C, 1–3 g daily, may be required to reduce risk of chronic diseases such as cardiovascular disease, cancer, or cataract (Carr and Frei, 1999a; Frei and Traber, 2001; Li and Schellhorn, 2007). Furthermore, the oxidative stress associated with many diseases may increase ascorbate requirements (Harman, 1994; Mezzetti et al., 1996). Large doses of ascorbate have been found to reduce cardiovascular disease risk, lengthen the lifespan of patients with cancer (Cameron and Pauling, 1979; Enstrom et al., 1992, Myint et al., 2008). Supporting this fact, the epidemiological studies have confirmed an inverse relationship between serum ascorbate level and blood pressure. A decrease in plasma vitamin C level has been observed in both type 1 and type 2 diabetes (Asard et al., 2004). Moreover, the literature has demonstrated that men might require more vitamin C than women (Jacob, 1990; VanderJagt et al., 1989) and that the elderly might require more ascorbate than younger people (Blanchard et al., 1990; Blanchard, 1991; Heseker and Schneider, 1994).
Safety of Vitamin C Supplementation
It has been suggested that vitamin C alone or mixed with N-acetyl-cysteine could be toxic, acting as a pro-oxidant (Podmore et al., 1998; Childs et al., 2001). However, the literature shows that ascorbic acid is not a pro-oxidant in vivo, even with iron co-supplementation (Carr and Frei, 1999a; Gomez-Cabrera et al., 2008).
The literature has also evoked the potential adverse effects of high doses of vitamin C, especially as regards the increase in oxalate and kidney stone formation (Levine et al., 1996; Levine et al., 1999). Indeed, Auer et al. demonstrated that 8 g/day, for 8 consecutive days, can cause harmful calcium oxalate crystalluria secondary to relative hyperoxaluria in persons who have a predisposition for increased crystal aggregation (Auer et al., 1998; Auer et al., 1998a). Wandzilak et al. observed a modest increase in urinary oxalate after administration of high doses of vitamin C (5 and 10 g/day for 5 days) (Wandzilak et al., 1994). Moreover, other work by Auer et al. using 4 g/day of ascorbic acid for 5 days, concluded that ingestion of these doses did not affect the principal risk factors associated with calcium oxalate kidney stone formation (Auer et al., 1998; Auer et al., 1998a). Furthermore, large doses of vitamin C (1.5 g or more) did not produce kidney stones and the doses of vitamin C above 1.5 g in fact reduced the risk of kidney stones (Curhan et al., 1996; Curhan et al., 1999; Gerster, 1997). Evidence indicates that high intakes of vitamin C do not increase oxalate excretion or induce the potential formation of kidney stones (Hathcock, 1997; Hathcock, 2005).
Gastrointestinal distress seems to be the most common adverse effect of higher doses of vitamin C intake (Miller and Hayes, 1982). When these symptoms occur, the vitamin C dosage is usually more than 2 g/day. The symptoms generally disappear within a week or 2with no further consequences, and may have been produced by other components such as sorbitol (Hill and Kamath, 1982).
Other studies and recent phase I clinical trials showed that large doses of vitamin C are safe (Hemilä, 1999; Hanck, 1982; Johnston, 1999; Garewal and Diplock, 1995; Riordan et al., 2005; Hoffer et al., 2008). A recent review demonstrated that vitamin C supplements of 2 g/day are safe for most adults (Hathcock et al., 2005). These authors also supported the claim that intakes of up to 4 g/day are well tolerated in the general population. No consistent and compelling data demonstrating serious adverse effects of vitamin C in humans have been established (Frei and Traber, 2001), although the tolerable upper limit intake has been estimated to 2 g/day (Food and Nutrition Board, 2000).
CONCLUSIONS
Vitamin C is an essential component of human physiology and should be supplied either through fresh fruits and vegetables or through supplements. The clinical benefit of vitamin C known so far is the prevention of scurvy. Intake of as little as 10 mg/day is sufficient for this purpose. In order to potentiate immune function or prevent chronic illnesses such as cancer, hypertension etc., higher doses of vitamin C are needed. Recent clinical updates on the role of vitamin C in tetanus (Hemilä and Koivula 2008) pneumonia (Hemilä and Louhiala 2007), asthma (Kaur et al., 2009), diabetic retinopathy (Lopes de Jesus et al., 2008) and pregnancy (Rumbold and Crowther 2005) are available as Cochrane reviews. Most of these reviews conclude that present knowledge does not allow a strong conclusions on the role of vitamin C, however the weakness of research methodology in vitamin C clinical trial has to be considered before further conclusion (Lykkesfeldt and Poulsen, 2009).
We need more research to explain some of the facts associated with vitamin C such as: lack of vitamin C supply causes decrease number of leukocytes, tobacco smoking lowers the plasma and leukocyte vitamin C level, refined carbohydrate seems to be accelerated the process of depleting vitamin C, women’s vitamin C plasma levels are approximately 20 % higher than men’s for any given dietary intake, vitamin C plasma level decreases by aging, etc. in order to get a clearer picture of the biological functions of vitamin C.
A recommendation of five servings of fruits and vegetables daily for the cancer prevention is not sufficient to obtain optimal benefit based on the current reviews. In addition, because of modern farming, handling and processing, more than 500 mg/day vitamin C would be difficult to obtain from dietary sources alone. It should be noted that pharmacokinetics and physiologic responses to vitamin C are known to vary considerably between individuals and optimal intakes for children, older adults and those suffering from acute and chronic diseases remains to be determined. Many studies have demonstrated that higher doses than the RDA for vitamin C can potentiate the immune system and prevent as well as treat a wide range of pathologies.
The need of vitamin C is higher to those who are in continuous oxidative stress. Oxidative stress is the focal point for chronic illnesses in human. The direct evidence and mechanism of action for the role of vitamin C in treating chronic illnesses could not be correlated yet, but results of epidemiologic and indirect studies are in strong support (Myint et al., 2008).
Consequently, even if vitamin C requirements vary greatly among individuals, it is suggested that vitamin C supplementation is not only safe but also necessary to achieve optimal health. Therefore, in agreement with the current literature, it can be suggested, especially to the older population to consume more than five servings of fruits and vegetables daily, added to 1 g of vitamin C supplementation divided in two or three doses during the day, in order toensure an optimal health. Moreover, male population compared to female population, older population compared to younger population, smoking population compared to non-smoking population and stressed population compared to non-stressed population may need higher consumption of vitamin C to obtain health beneficial effects.
Conflict of interest: None
References
Ames BN. A role for supplements in optimizing health: The metabolic tune-up. Arch Biochem Biophys 2004; 423: 227-234.
Ames BN. Increasing longevity by tuning up metabolism: To maximize human health and lifespan, scientists must abandon outdated models of micronutrients. EMBO Rep 2005; 6: S20-S24.
Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA 2006; 103: 17589-17594.
Anderson R. Ascorbate-mediated stimulation of neutrophil motility and lymphocyte transformation by inhibition of the peroxidase/H2O2/halide system in vitro and in vivo. Am J Clin Nutr 1981; 34: 1906-1911.
Anderson R. Assessment of oral ascorbate in three children with chronic granulomatous disease and defective neutrophil motility over a 2-year period. Clin Exp Immunol 1981a; 43: 180-188.
Anderson R. Effects of ascorbate on normal and abnormal leukocyte functions. In: Vitamin C: New Clinical Applications in Immunology, Lipid Metabolism and Cancer. A. Hank Huber, Bern: Germany 1982; pp. 23-24.
Asard H, May JM, Smirnoff N (Ed). Vitamin C: Functions and biochemistry in animals and plants BIOS Scientific Publisher, Taylor and Francis Group, London and NewYork, 2004; p 60.
Auer BL, Auer D, Rodgers AL. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur J Clin Invest 1998; 28: 695-700.
Auer BL, Auer D, Rodgers AL. The effect of ascorbic acid ingestion on the biochemical and physicochemical risk factors associated with calcium oxalate kidney stone formation. Clin Chem Lab Med 1998a; 36: 143-147.
Benade L, Howard T, Burk D. Synergistic killing of Ehrlich ascites carcinoma cells by ascorbate and 3-amino-1,2,4,-triazole. Oncology 1969; 23: 33-43.
Bendich A, Langseth L. The health effects of vitamin C supplementation: A review. J Am Coll Nutr 1995; 14: 124-136.
Blanchard J, Conrad KA, Mead RA et al. Vitamin C disposition in young and elderly men. Am J Clin Nutr 1990; 51: 837-845.
Blanchard J. Depletion and repletion kinetics of vitamin C in humans. J Nutr 1991; 121: 170-176.
Bram S, Froussard P, Guichard M et al. Vitamin C preferential toxicity for malignant melanoma cells. Nature 1980; 284: 629-631.
Cameron E, Pauling L. Ascorbic acid and glucosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology 1973; 27: 181-192.
Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem-Biol Interact 1974; 9: 285-315.
Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA 1976; 73: 3685-3689.
Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA 1978; 75: 4538-4542.
Cameron E, Pauling L. Cancer and Vitamin C. Palo Alto, CA: Linus Pauling Institute of Science and Medicine, 1979.
Cameron E, Pauling L. Cancer and Vitamin C. Philadelphia: Camino Books, 1993.
Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999; 13: 1007-1024.
Carr A, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 1999a; 69: 1086-1107.
Casciari JJ, Riordan NH,Schmidt TL et al. Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumors. Br J Cancer 2001; 84: 1544-1550.
Challem JJ. Toward a new definition of essential nutrients: Is it now time for a third “vitamin” paradigm? Med Hypotheses 1999; 52: 417-422.
Chen Q, Espey MG, Krishna MC et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 2005; 102: 13604-13609.
Chen Q, Espey MG, Sun AY et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 2007; 104: 8749-8754.
Chen Q, Espey MG, Sun AY et al. Pharmacological doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 2008; 105: 11105-11109.
Childs A, Jacobs C, Kaminski T et al. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med 2001; 31: 745-753.
Creagan ET, Moertel CG, O’Fallon JR et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979; 301: 687-690.
Curhan GC, Willett WC, Rimm EB et al. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol 1996; 155: 1847-1851.
Curhan GC, Willett WC, Speizer FE et al. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 1999; 10: 840-845.
Davies K. The broad spectrum of responses to oxidants in proliferating cells: A new paradigm for oxidative stress. IUBMB Life 1999; 48: 41-47.
Dawson EB, Evans DR, Harris WA et al. The effect of ascorbic acid supplementation on the blood lead levels of smokers. J Am Coll Nutr 1999; 18: 166-170.
De Rosa SC, Zaretsky MD, Dubs JG et al. N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clin Invest 2000; 30: 915-929.
Deruelle F, Baron B. Vitamin C: Is supplementation necessary for optimal health? J Alt Compl Med 2008; 14: 1291-1298.
Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr 1986; 6: 365-406.
Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 1992; 3: 194-202.
Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: Findings from the nutrition addon study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Am J Clin Nutr 2003; 78: 999-1010.
Food and Nutrition Board IoM. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academy Press, 2000; 95-185.
Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989; 86: 6377-6381.
Frei B, Traber MG. The new US Dietary Reference Intakes for vitamins C and E. Redox Rep 2001; 6: 5-9.
Frei B, Lawson S. Vitamin C and cancer revisited. Proc Natl Acad Sci USA 2008; 105: 11037-11038.
Garewal HS, Diplock AT. How ‘safe’ are antioxidant vitamins? Drug Saf 1995; 13: 8-14.
Gerster H. No contribution of ascorbic acid to renal calcium oxalate stones. Ann Nutr Metab 1997; 41: 269-282.
Gil MI, Ferreres F, Tomas-Barberan FA. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids andvitamin C) of fresh-cut spinach. J Agric Food Chem 1999; 47: 2213-2217.
Gomez-Cabrera MC, Domenech E, Romagnoli M et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 2008; 87: 142-149.
Graumlich JF, Ludden TM, Conry-Cantilena C et al. Pharmacokinetic model of ascorbic acid in healthy male volunteers mduring depletion and repletion. Pharm Res 1997; 14: 1133-1139.
Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing – where do we stand? Frontiers in Bioscience 2008; 13: 6554-6579.
Guenther PM, Dodd KW, Reedy J et al. Most Americans eat much less than recommended amounts of fruits and vegetables. J Am Diet Assoc 2006; 106: 1371-1379.
Halliwell B. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radical. Anal Biochem 1987; 165: 215-219.
Hanck A. Tolerance and effects of high doses of ascorbic acid: Dosis facit venenum. Int J Vitam Nutr Res 1982; 23(suppl): 221–238.
Harman D. Ageing: a theory based on free-radical and radiation chemistry. J Gerentol 1956; 11: 298-300.
Harman D. Free-radical theory of aging. Increasing the functional life span. Ann N Y Acad Sci 1994; 717: 1-15.
Hathcock JN, Azzi A, Blumberg J et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 2005; 81: 736-745.
Hathcock JN. Vitamins and minerals: Efficacy and safety. Am J Clin Nutr 1997; 66: 427-437.
Haworth WN, Hirst EL. Synthesis of ascorbic acid. J Soc Chem Ind 1933; 52: 645-647.
Hemilä H. Vitamin C and the common cold. Br J Nutr 1992; 67: 3-16.
Hemilä H. Vitamin C supplementation and common cold symptoms: Problems with inaccurate reviews. Nutrition 1996; 12: 804-809.
Hemilä H. Vitamin C supplementation and common cold symptoms: Factors affecting the magnitude of the benefit. Med Hypotheses 1999; 52: 171-178.
Hemilä H. Vitamin C supplementation and respiratory infections: A systematic review. Mil Med 2004; 169: 920–925.
Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database of Syst Rev 2007, Issue 1. Article no. CD005532.
Hemilä H, Chalker E, Treacy B et al. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2007a: CD000980.
Hemilä H, Koivula T. Vitamin C for preventing and treating tetanus. Cochrane Database of Syst Rev 2008, Issue 2. Article no. CD006665.
Heseker H, Schneider R. Requirement and supply of vitamin C, E and beta-carotene for elderly men and women. Eur J Clin Nutr 1994; 48: 118-127.
Hill RE, Kamath KR. “Pink” diarrhoea: Osmotic diarrhoea from a sorbitol-containing vitamin C supplement. Med J Aust 1982; 1: 387-389.
Hoffer A, Pauling L. Hardin Jones biostatistical analysis of mortality data for a second set of cohorts of cancer patients with a large fraction surviving at the termination of the study and a comparison of survival times of cancer patients receiving large regular oral doses of vitamin C and other nutrients with similar patients not receiving these doses. Orthomol Med 1993; 8: 157-167.
Hoffer LJ, Levine M, Assouline S et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 2008; 19: 1969-1974.
Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci 1975; 258: 103-118.
Jacob RA. Assessment of human vitamin C status. J Nutr 1990; 120(suppl 11): 1480-1485.
Jacob RA, Kelley DS, Pianalto FS et al. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr 1991; 54: 1302S-1309S.
Johnston CS. Biomarkers for establishing a tolerable upper intakelevel for vitamin C. Nutr Rev 1999; 57: 71-77.
Johnston CS, Bowling DL. Stability of ascorbic acid in commercially available orange juices. J Am Diet Assoc 2002; 102: 525–529.
Kaur B, Rowe BH, Arnold E. Vitamin C supplementation for asthma. Cochrane Database of Syst Rev 2009, Issue 1. Article no. CD000993.
Kennes B, Dumont I, Brohee D et al. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology 1983; 29: 305-310.
Kharb S, Singh V, Ghalaut PS et al. Glutathione levels in health and sickness. Indian J Med Sci 2000; 54: 52-54.
Knight JA. Free radicals. Their history and current status in aging and disease. Ann Clin Lab Sci 1998; 28: 331-346.
Lachance P, Langseth L. The RDA concept: Time for a change? Nutr Rev 1994; 52: 266-270.
Lenton KJ, Sane AT, Therriault H et al. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am J Clin Nutr 2003; 77: 189-195.
Leung PY, Miyashita K, Young M et al. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res 1993; 13: 475-480.
Levine M, Dhariwal KR, Welch RW et al. Determination of optimal vitamin C requirements in humans. Am J Clin Nutr 1995; 62: 1347S-1356S.
Levine M, Conry-Cantilena C, Wang Y et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996; 93: 3704-3709.
Levine M, Rumsey SC, Daruwala R et al. Criteria and recommendations for vitamin C intake. JAMA 1999; 281: 1415-1423.
Levine M, Wang Y, Padayatty SJ et al. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA 2001; 98: 9842-9846.
Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007; 137: 2171-2184.
Lind J. A Treatise of the Scurvy in Three Parts, Containing an inquiry into the nature, causes and cure of that disease, together with a critical and chronological view of what has been published on the subjects. A Millar, London 1753.
Linster CL, Van Schaftingen E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J 2007; 274: 1-22.
Ljubuncic P, Reznick AZ. The evolutionary theories of aging revisited – a mini-review. Gerontology 2009; 55: 205-216.
Lopes de Jesus CC, Atallah AN, Valente O, Trevisani VFM. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database of Syst Rev 2008, Issue 1. Article no. CD006695.
Loria CM, Klag MJ, Caulfield LE et al. Vitamin C status and mortality in US adults. Am J Clin Nutr 2000; 72: 139-145.
Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised control trails. Brit J Nutr 2009 doi: 10.1017/S0007114509993229.
Maggini S, Wintergerst ES, Beveridge S et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 2007; 98(suppl 1): S29-S35.
Mandl J, Szark A, Banhegyi G. Vitamin C: update physiology and pharmacology. Brit J Pharmacol 2009; 157: 1097-1110.
May JM, Huang J, Qu ZC. Macrophage uptake and recycling of ascorbic acid: Response to activation by lipopolysaccharide. Free Rad Biol Med 2005; 39: 1449-1459.
McCord JM, Fridovich I. Superoxide dismutase. An enzyme function for erythrocuprein (hemocuprein). J Biol Chem 1969; 244: 6049-6055.
Mezzetti A, Lapenna D, Romano F et al. Systemic oxidative stress and its relationship with age and illness. Associazione Medica “Sabin”. J Am Geriatr Soc 1996; 44: 823-827.
Miller DR, Hayes KC. Vitamin excess and toxicity. In: Hathcock JN, ed. Nutritional Toxicology. Vol 1. New York: Academic Press, 1982: 81-133.
Moertel CG, Fleming TR, Creagan ET et al. High-dose vitaminC versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: A randomized double-blind comparison. N Engl J Med 1985; 312: 137-141.
Moser U. Uptake of ascorbic acid by leukocytes. Ann N Y Acad Sci 1987; 498: 200-215.
Myint PK, Luben RN, Welch AA et al. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20649 participants of the European Prospective Investigation into Cancer–Norfolk prospective population study. Am J Clin Nutr 2008; 87: 64-69.
Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol 1997; 12: 525-535.
Omaye ST, Skala JH, Jacob RA. Plasma ascorbic acid in adult males: Effects of depletion and supplementation. Am J Clin Nutr 1986; 44: 257-264.
Padayatty SJ, Sun H, Wang Y et al. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med 2004; 140: 533-537.
Padayatty SJ, Riordan HD, Hewitt SM et al. Intravenously administered vitamin C as cancer therapy: Three cases. CMAJ 2006; 174: 937-942.
Pauling L. Vitamin C and the common cold. W.H. Freeman, San Francisco, CA. 1970.
Pauling L. Are recommended daily allowances for vitamin C adequate? Proc Natl Acad Sci USA 1974; 71: 4442-4446.
Podmore ID, Griffiths HR, Herbert KE et al. Vitamin C exhibits pro-oxidant properties. Nature 1998; 392: 559.
Rattan SI. Theories of biological aging: Genes, proteins, and free radicals. Free Rad Res 2006; 40() 1230-1238.
Riordan NH, Riordan HD, Meng X et al. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses 1995; 44: 207-213.
Riordan HD, Casciari JJ, Gonzalez MJ et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. Puerto Rico Health Sci J 2005; 24: 269-276.
Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 1. Article no. CD004072.
Severi S, Bedogni G, Zoboli GP et al. Effects of home-based food preparation practices on the micronutrient content of foods. Eur J Cancer Prev 1998; 7: 331-335.
Svirbely JL, Szent-Györgyi A. Hexuronic acid as the antiscorbutic factor. Nature 1932; 129: 576.
VanderJagt DJ, Garry PJ, Bhagavan HN. Ascorbate and dehydroascorbate: Distribution in mononuclear cells of healthy elderly people. Am J Clin Nutr 1989; 49: 511-516.
Verrax J, Calderon PB. The controversial place of vitamin C in cancer treatment. Biochem Pharmacol 2008; 76: 1644-1652.
Viña J, Borrás C, Miquel J. Theories of ageing. IUBMB Life 2007; 59: 249-254.
Wandzilak TR, D’Andre SD, Davis PA et al. Effect of high dose vitamin C on urinary oxalate levels. J Urol 1994; 151: 834-837.
Washko P, Rotrosen D, Levine M. Ascorbic acid in human neutrophils. Am J Clin Nutr 1991; 54: 1221S-1227S.
Wolf G. Uptake of ascorbic acid by human neutrophils. Nutr Rev 1993; 51: 337-338.
Manuscript received October 7, 2009 and accepted April 30, 2010.
Norsk Farmaceutisk Tidsskrift 2010; 9: 20–4.
